Content provided via multiple sources : including UptoDate
Coronavirus : CoVid – 19
Last Updated : September 09, 2021
The ongoing management of Covid is complicated by the multiple clinical pathways and symptoms patient report. Everyone should speak with their doctor about their specific concerns. I have updated a few medications which are often requested or which questions are asked. These are the national guidelines which parallel my recommendations moving forward. I will update this as topic as time moves on.
Colchicine – Although there are some data demonstrating a benefit from the use of colchicine in early, mild to moderate COVID-19, the benefit is modest without a reduction in mortality, and adverse effects are common. In a randomized trial including over 4100 adult outpatients (≥age 40) with early, mild to moderate, testing-confirmed COVID-19, treatment with oral colchicine (0.5 mg twice daily for three days, followed by 0.5 mg daily for a total of 30 days), reduced the risk of hospitalization compared with placebo (4.5 versus 5.9 percent of patients; odds ratio [OR] 0.75, 95% CI 0.57-0.99). However, there was no reduction in mortality. Gastrointestinal side effects (eg, diarrhea) were more common, and pulmonary embolism occurred more frequently in the colchicine compared with the placebo group (24 versus 15 percent; and 0.5 versus 0.1 percent, respectively).
●Ivermectin – As with other interventions that do not have a clear benefit, we recommend that ivermectin not be used for treatment of COVID-19 outside a clinical trial. Several meta-analyses have highlighted that the effect of ivermectin in patients with COVID-19 remains uncertain because of a lack of high-quality data. As an example, in a July 2021 meta-analysis that identified four trials comparing ivermectin with placebo or standard care in outpatients with mild COVID-19, there was no clear reduction in all-cause mortality at 28 days (RR 0.33 in two trials, 95% CI 0.01-8.05), no reduction in need for invasive mechanical ventilation at 14 days (RR 2.97 in one trial; 95% CI 0.12-72.47), and no clear impact on symptom resolution at 14 days (RR 1.04 in one trial, 95% CI 0.89-1.21). The quality of the evidence for these outcomes was deemed low to very low because of imprecision and risk of bias. Although the meta-analysis also did not identify any increased risk of adverse effects with ivermectin, the CDC has subsequently reported a steep increase in calls to poison control centers about ivermectin toxicity compared with pre-pandemic rates. Several of these calls involved ivermectin obtained without prescription (eg, from internet or veterinary sources); some patients were hospitalized for neurologic adverse effects related to uncertain dosages.
●Fluvoxamine – Limited data suggest that fluvoxamine may reduce progression to severe disease in early, mild COVID-19, although high-quality evidence is lacking. As examples, in a small randomized controlled trial including 152 outpatients with nonsevere COVID-19, treatment with fluvoxamine (100 mg orally twice daily for two days, then three times daily for a total of 15 days of treatment) reduced clinical (respiratory) deterioration compared with placebo (0 versus 8.3 percent; ARR 8.7, 95% CI 1.8-16.4). The trial, however, was limited by methodologic problems, including limited study duration and loss to follow-up. In a prospective cohort study including 113 adults with asymptomatic or mild COVID-19, treatment with fluvoxamine (50 mg twice daily for 14 days) was associated with a reduced incidence of hospitalization compared with observation alone (0 versus 12.5 percent). In addition, persistent symptoms were less likely among those treated with fluvoxamine at 14 days (0 versus 60 percent), although this observation is limited by the short duration of follow-up.
●Hydroxychloroquine and azithromycin – Hydroxychloroquine and azithromycin have received attention as agents with possible antiviral activity, but trials have not suggested a clinical benefit for patients with COVID-19, including those managed in the outpatient setting. Although some observational and unpublished anecdotal reports have suggested a clinical benefit of hydroxychloroquine, those are subject to a number of potential confounders, and randomized trials offer higher-quality evidence that hydroxychloroquine has no proven role for COVID-19. As an example, in an open-label trial including 293 patients with mild COVID-19 who did not warrant hospitalization, hydroxychloroquine administered within five days of symptom onset did not reduce viral levels at day 3 or 7 compared with no treatment. In addition, there was no statistically significant reduction in hospitalization rates or time to symptom resolution. The rate of adverse effects, primarily gastrointestinal symptoms, were greater with hydroxychloroquine.
__________________________________________________________________________________________________________________________________________
Globally, more than 12 million confirmed cases of COVID-19 have been reported. Updated case counts in English can be found on the World Health Organization and European Centre for Disease Prevention and Control websites. An interactive map highlighting confirmed cases throughout the world can be found here.
Since the first reports of cases from Wuhan, a city in the Hubei Province of China, at the end of 2019, cases have been reported in all continents, except for Antarctica.
In the United States, COVID-19 has been reported in all 50 states, Washington DC, and at least four territories. The cumulative incidence varies by state and likely depends on a number of factors, including population density and demographics, extent of testing and reporting, and timing of mitigation strategies. In the United States, outbreaks in long-term care facilities and homeless shelters have emphasized the risk of exposure and infection in congregate settings.
The reported case counts underestimate the overall burden of COVID-19, as only a fraction of acute infections are diagnosed and reported.
The rate of prior exposure to SARS-CoV-2, as reflected by seropositivity, exceeds the incidence of reported cases by approximately 10-fold.
Content obtained from UpToDate
CoVid -19 Information
Transission – Understanding of the transmission risk is incomplete. Epidemiologic investigation in Wuhan at the beginning of the outbreak identified an initial association with a seafood market that sold live animals, where most patients had worked or visited and which was subsequently closed for disinfection. However, as the outbreak progressed, person-to-person spread became the main mode of transmission.
Route of person-to-person transmission — Direct person-to-person transmission is the primary means of transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is thought to occur through close-range contact, mainly via respiratory droplets; virus released in the respiratory secretions when a person with infection coughs, sneezes, or talks can infect another person if it makes direct contact with the m

SARS-CoV-2 has been detected in non-respiratory specimens, including stool, blood, ocular secretions, and semen, but the role of these sites in transmission is uncertain. In particular, several reports have described detection of SARS-CoV-2 RNA from stool specimens, even after viral RNA could no longer be detected from upper respiratory specimens, and live virus has been cultured from stool in rare cases.
Detection of SARS-CoV-2 RNA in blood has also been reported in some but not all studies that have tested for it. However, the likelihood of bloodborne transmission (eg, through blood products or needlesticks) appears low; respiratory viruses are generally not transmitted through the bloodborne route, and transfusion-transmitted infection has not been reported for SARS-CoV-2 or for the related Middle East respiratory syndrome coronavirus (MERS-CoV) or SARS-CoV .
There is also no evidence that SARS-CoV-2 can be transmitted through contact with non-mucous membrane sites (eg, abraded skin).
Period of greatest infectiousness – Largely indirect data suggest that infected individuals are more likely to be infectious in the earlier stages of infection. Viral RNA levels from upper respiratory specimens appear to be higher soon after symptom onset compared with later in the illness
Transmission of SARS-CoV-2 from asymptomatic individuals (or individuals within the incubation period) has also been well documented. The biologic basis for this is supported by a study of a SARS-CoV-2 outbreak in a long-term care facility, in which infectious virus was cultured from reverse transcription polymerase chain reaction (RT-PCR)-positive upper respiratory tract specimens in presymptomatic and asymptomatic patients as early as six days prior to the development of typical symptoms. However, the extent to which asymptomatic or presymptomatic transmission occurs and how much it contributes to the pandemic remain unknown.
Duration of infectiousness and significance of viral RNA shedding – How long a person remains infectious is also uncertain, but available data suggest that prolonged viral RNA shedding after symptom resolution is not clearly associated with prolonged infectiousness. The duration of viral RNA shedding is variable; there appears to be a wide range, which may depend on severity of illness.
Risk of transmission depends on exposure type — The risk of transmission from an individual with SARS-CoV-2 infection varies by the type and duration of exposure, use of preventive measures, and likely individual factors (eg, the amount of virus in respiratory secretions).
The risk of transmission after contact with an individual with COVID-19 increases with the closeness and duration of contact and appears highest with prolonged contact in indoor settings. Thus, most secondary infections have been described in the following settings:
●Among household contacts
●In health care settings when personal protective equipment was not used.
●In other congregate settings where individuals are residing or working in close quarters (eg, cruise ships, homeless shelters, and detention facilities)
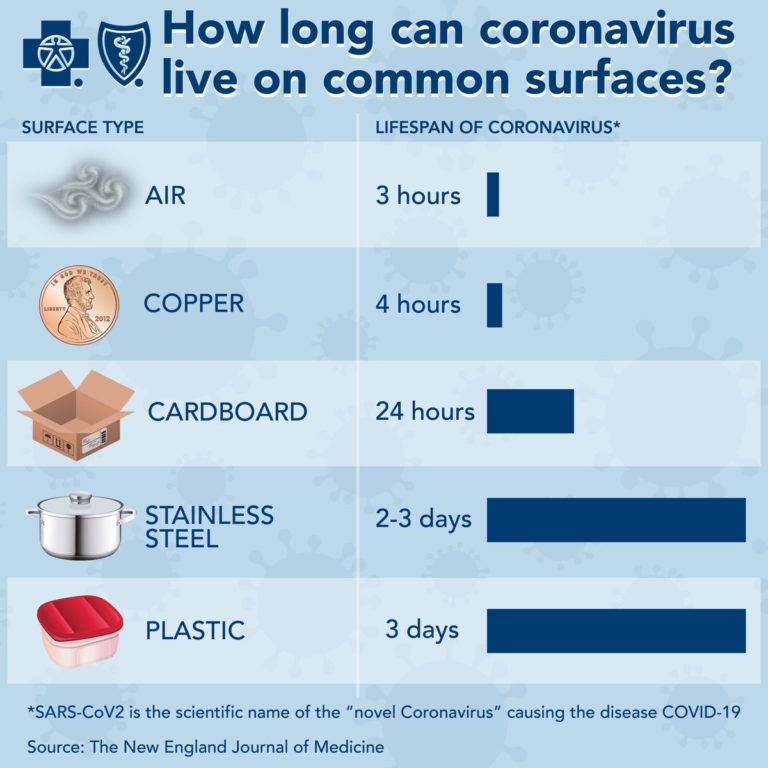
Environmental contamination — Virus present on contaminated surfaces may be another source of infection if susceptible individuals touch these surfaces and then transfer infectious virus to mucous membranes in the mouth, eyes, or nose. The frequency and relative importance of this type of transmission remain unclear. It may be more likely to be a potential source of infection in settings where there is heavy viral contamination (eg, in an infected individual’s household or in health care settings).
It is unknown how long SARS-CoV-2 can persist on surfaces; other coronaviruses have been tested and may survive on inanimate surfaces for up to six to nine days without disinfection.
In a study evaluating the survival of viruses dried on a plastic surface at room temperature, a specimen containing SARS-CoV (a virus closely related to SARS-CoV-2) had detectable infectivity at six but not nine days. However, in a systematic review of similar studies, various disinfectants (including ethanol at concentrations between 62 and 71%) inactivated a number of coronaviruses related to SARS-CoV-2 within one minute. Simulated sunlight has also been shown to inactivate SARS-CoV-2 over the course of 15 to 20 minutes in experimental conditions, with higher levels of ultraviolet-B (UVB) light associated with more rapid inactivation. Based on data concerning other coronaviruses, duration of viral persistence on surfaces also likely depends on the ambient temperature, relative humidity, and the size of the initial inoculum.
Immunity and risk of reinfection — Antibodies to the virus are induced in those who have become infected. Preliminary evidence suggests that some of these antibodies are protective, but this remains to be definitively established. Moreover, it is unknown whether all infected patients mount a protective immune response and how long any protective effect will last.
The FDA has granted emergency use authorization for tests that identify antibodies against SARS-CoV-2 in serum or plasma. Should evidence confirm that the presence of these antibodies reflects a protective immune response, serologic screening will be an important tool to understand population immunity and distinguish individuals who are at lower risk for reinfection.

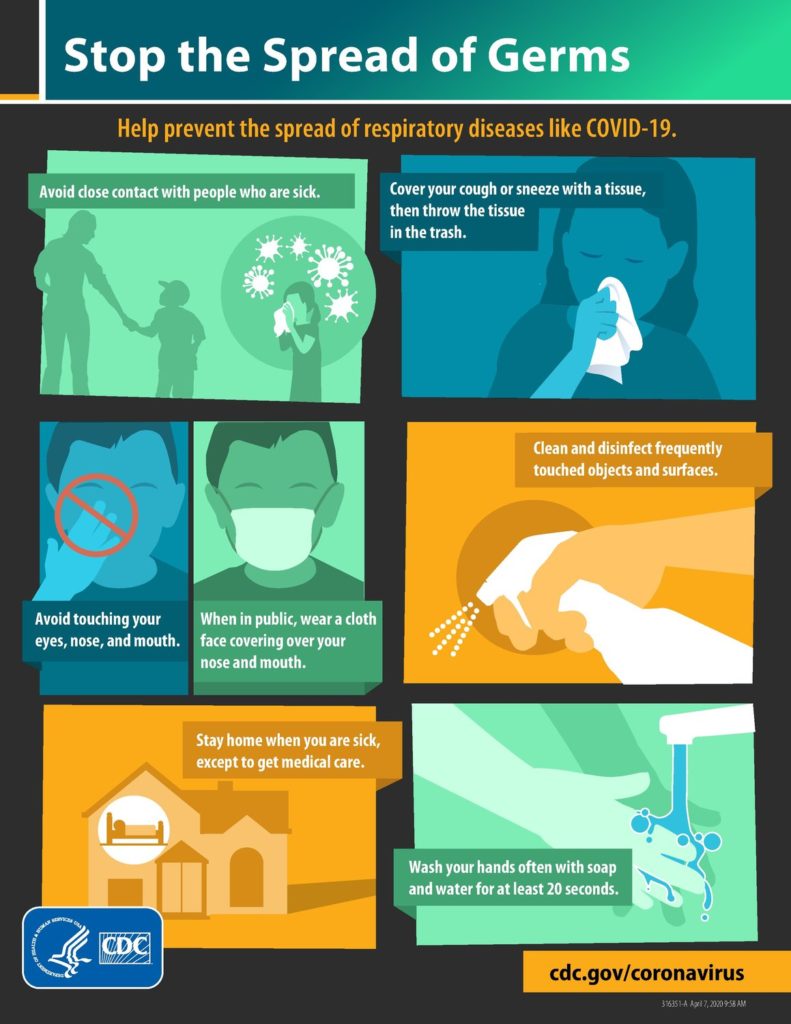
Personal preventive measures — If community transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is present, residents should be encouraged to practice social distancing by avoiding crowds and maintaining a distance of six feet (two meters) from others when in public. In particular, individuals should avoid close contact with ill individuals. Individuals are also encouraged to wear masks when out in public. These and other general public health measures are discussed elsewhere.
The following general measures are additionally recommended to reduce transmission of infection:
●Diligent hand washing, particularly after touching surfaces in public. Use of hand sanitizer that contains at least 60 percent alcohol is a reasonable alternative if the hands are not visibly dirty.
●Respiratory hygiene (eg, covering the cough or sneeze).
●Avoiding touching the face (in particular eyes, nose, and mouth). The American Academy of Ophthalmology suggests that people not wear contact lenses, because they make people touch their eyes more frequently.
●Cleaning and disinfecting objects and surfaces that are frequently touched. The United States Centers for Disease Control and Prevention (CDC) has issued guidance on disinfection in the home setting; a list of Environmental Protection Agency-registered products can be found here.
●Ensure adequate ventilation of indoor spaces.
These measures should be followed by all individuals, but should be emphasized for older adults and individuals with chronic medical conditions, in particular.
The rationale for all individuals (regardless of symptoms) to wear a mask or face covering in the community is primarily to contain secretions of and prevent transmission from individuals with infection, including those who have asymptomatic or presymptomatic infection.
Individuals who develop an acute respiratory illness (eg, with fever and/or respiratory symptoms) or other symptoms consistent with COVID-19 should be encouraged to self-isolate at home (away from other individuals and pets in the household) for the duration of the illness and wear a mask or face covering if they have to be around other people.
Some may warrant evaluation for COVID-19. Individuals who are caring for patients with suspected or documented COVID-19 at home should also wear a face cover when in the same room as that patient.
Other public health measures
On January 30, 2020, the WHO declared the COVID-19 outbreak a public health emergency of international concern and, in March 2020, began to characterize it as a pandemic in order to emphasize the gravity of the situation and urge all countries to take action in detecting infection and preventing spread. Throughout the world, countries have employed various nonpharmaceutical interventions to reduce transmission. In addition to personal preventive measures (eg, hand hygiene, respiratory etiquette and face covers, environmental disinfection), transmission reduction strategies include:
●Social/physical distancing orders
●Stay-at-home orders
●School, venue, and nonessential business closure
●Bans on public gatherings
●Travel restriction with exit and/or entry screening
●Aggressive case identification and isolation (separating individuals with infection from others)
●Contact tracing and quarantine (separating individuals who have been exposed from others)
Managing asymptomatic individuals with potential exposure — In areas where SARS-CoV-2 is prevalent, all residents should be encouraged to stay alert for symptoms and practice social distancing by staying home as much as possible and maintaining six feet (two meters) distance from others when they have to leave the home.
In the United States, the CDC suggests this approach for all residents. For those returning from international travel (including cruise ship travel) and those who have had close contact with a patient with suspected or confirmed COVID-19 (including during the 48 hours prior to that patient developing symptoms), the CDC also suggests:
●Self-quarantine at home for 14 days following the last exposure, with maintenance of at least six feet (two meters) from others at all times.
●Avoiding contact with individuals at high risk for severe illness (unless they are household members with the same exposure).
●Twice-daily temperature checks with monitoring for fever, cough, or dyspnea. If they develop such clinical manifestations, they should continue to stay at home away from other household members and contact their medical providers.

“CDC LINK” will take you to the CDC website “High Risk Populations” page to determine if you or a family member is “At high risk for CoVid complications”
People of any age with certain underlying medical conditions are at increased risk for severe illness from COVID-19
People of any age with the following conditions are at increased risk of severe illness from COVID-19:
The Green Button will direct you to the CDC recommendations regarding home management.
Follow Up Care :
Most patients discharged from the inpatient setting warrant clinician follow-up within one to two days following discharge; whether a telehealth or in-person outpatient visit is most appropriate depends on their unique clinical and social situation. Temporary housing in supervised residential care facilities, when available, may also be appropriate for some patients discharged from the inpatient hospital setting.